Conditioned medium-enriched umbilical cord mesenchymal stem cells: a potential therapeutic strategy for spinal cord injury, unveiling transcriptomic and secretomic insights
Rajasekaran Subbarayan 1, Dinesh Murugan Girija 2, Selvaraj Thirupathi Kumara Raja 3, Alagudurai Krishnamoorthy 4, Dhasarathdev Srinivasan 1, Rupendra Shrestha 5, Nityanand Srivastava 6, Suresh Ranga Rao 7
- Centre for Advanced Biotherapeutics and Regenerative Medicine, Research‑FAHS, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam 603013, India
- Vopec Pharmaceuticals Pvt Limited, Chennai, India
- School of Chemical and Biotechnology, SASTRA Deemed University, Thanjavur, India
- International Institute of Biotechnology and Toxicology (IBAT), Chennai, India
- Research and Collaboration, Anka Analytica, Melbourne, Australia
- Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY, USA
- Department of Engineering Design, Indian Institute of Technology Madras, Chennai, India
Publication: Molecular Biology Reports.2024 Apr 24;51(1):570. doi:10.1007/s11033-024-09503-8
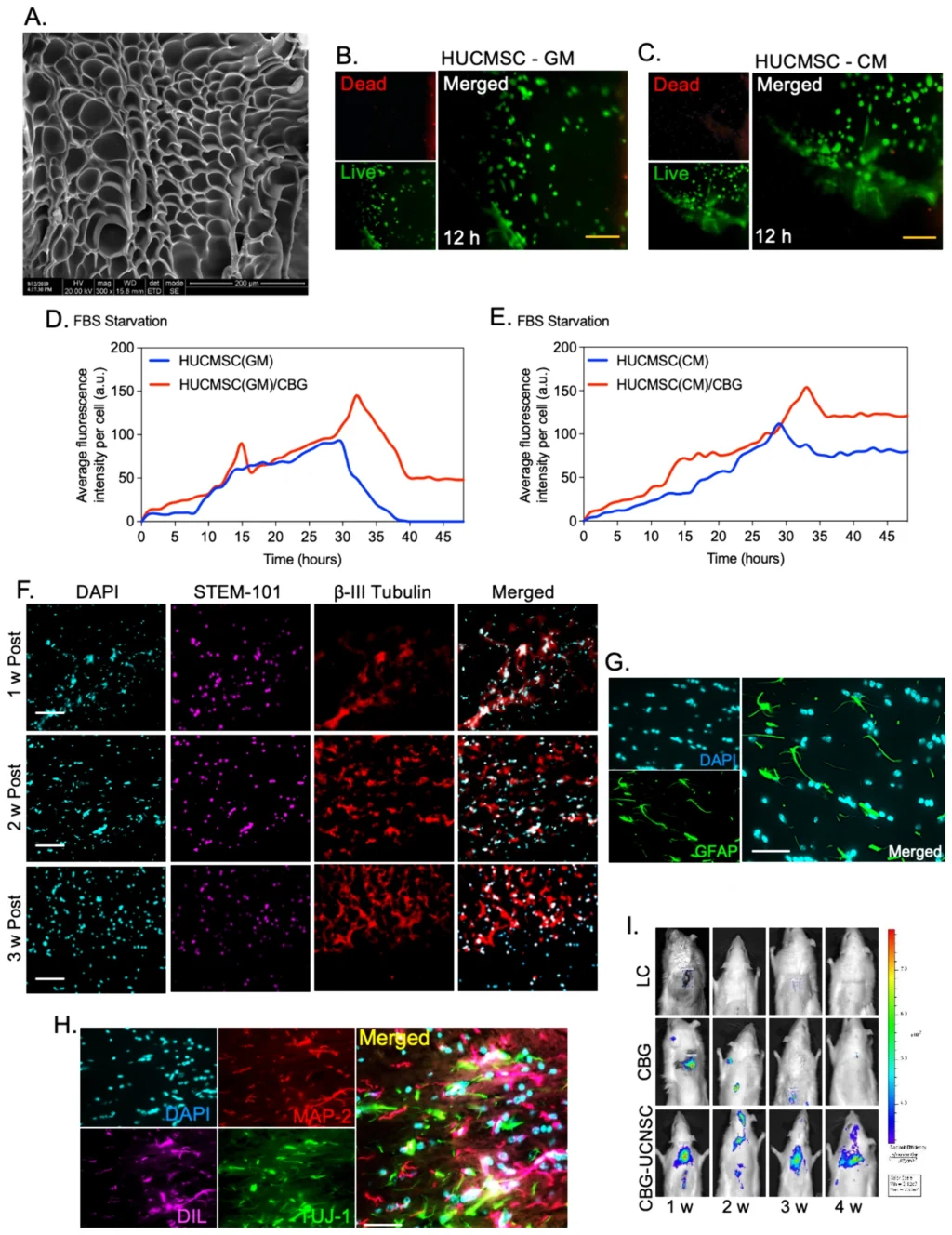
Abstract – Introduction: Spinal cord injury (SCI) leads to significant destruction of nerve tissue, causing the degeneration of axons and the formation of cystic cavities. This study aimed to examine the characteristics of human umbilical cord-derived mesenchymal stem cells (HUCMSCs) cultured in a serum-free conditioned medium (CM) and assess their effectiveness in a well-established hemitransection SCI model. Material and Methods: In this study, HUCMSCs cultured medium was collected and characterized by measuring IL-10 and identifying proteomics using mass spectroscopy. This collected serum-free CM was further used in the experiments to culture and characterize the HUMSCs. Later, neuronal cells derived from CM-enriched HUCMSC were tested sequentially using an injectable caffeic acid-bioconjugated gelatin (CBG), which was further transplanted in a hemitransection SCI model. In vitro, characterization of CM-enriched HUCMSCs and differentiated neuronal cells was performed using flow cytometry, immunofluorescence, electron microscopy, and post-transplant analysis using immunohistology analysis, qPCR, in vivo bioluminescence imaging, and behavioral analysis using an infrared actimeter. Results: The cells that were cultured in the conditioned media produced a pro-inflammatory cytokine called IL-10. Upon examining the secretome of the conditioned media, the Kruppel-like family of KRAB and zinc-finger proteins (C2H2 and C4) were found to be activated. Transcriptome analysis also revealed an increased expression of ELK-1, HOXD8, OTX2, YY1, STAT1, ETV7, and PATZ1 in the conditioned media. Furthermore, the expression of Human Stem-101 confirmed proliferation during the first 3 weeks after transplantation, along with the migration of CBG-UCNSC cells within the transplanted area. The gene analysis showed increased expression of Nestin, NeuN, Calb-2, Msi1, and Msi2. The group that received CBG-UCNSC therapy showed a smooth recovery by the end of week 2, with most rats regaining their walking abilities similar to those before the spinal cord injury by week 5. Conclusions: In conclusion, the CBG-UCNSC method effectively preserved the integrity of the transplanted neuronal-like cells and improved locomotor function. Thus, CM-enriched cells can potentially reduce biosafety risks associated with animal content, making them a promising option for clinical applications in treating spinal cord injuries.
Cite: Subbarayan R, Murugan Girija D, Raja STK, Krishnamoorthy A, Srinivasan D, Shrestha R, Srivastava N, Ranga Rao S. Conditioned medium-enriched umbilical cord mesenchymal stem cells: a potential therapeutic strategy for spinal cord injury, unveiling transcriptomic and secretomic insights. Mol Biol Rep. 2024 Apr 24;51(1):570. PMID: 38658405.