Molecular insights on Eltrombopag: potential mitogen stimulants, angiogenesis, and therapeutic radioprotectant through TPO-R activation
Rajasekaran Subbarayan 1, Dhasarathdev Srinivasan 1, Salman Sadullah Usmani 2, Dinesh Murugan Girija 3, Shoeb Ikhlas 4, Nityanand Srivastav 4, Ranjith Balakrishnan 1, Rupendra Shrestha 5, Ankush Chauhan 6
- Centre for Advanced Biotherapeutics and Regenerative Medicine, Research-FAHS, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, India.
- Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY, USA.
- Research and Development, Vopec Pharmaceuticals Pvt Limited, Chennai, India.
- Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY, USA.
- Research and Collaboration, Anka Analytica, Melbourne, Australia.
- Centre for Herbal Pharmacology and Environmental Sustainability, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, India.
Publication: Platelets. 2024 Dec;35(1):2359028. doi:10.1080/09537104.2024.2359028
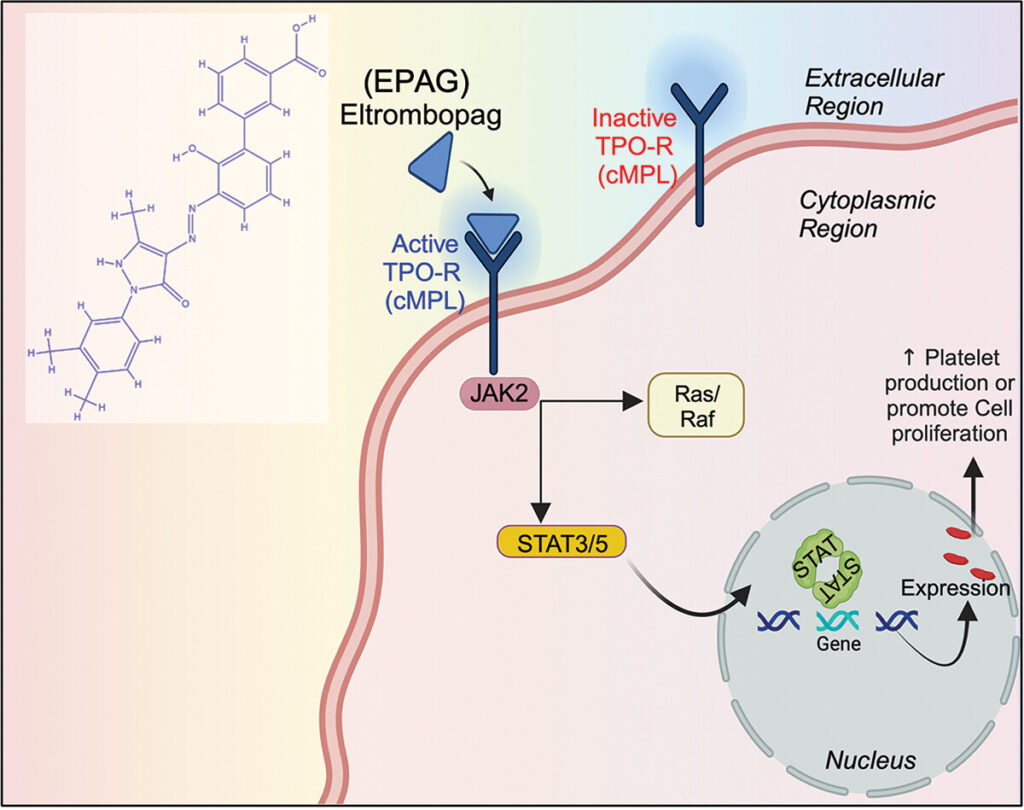
Abstract – The purpose of this study is to investigate the molecular interactions and potential therapeutic uses of Eltrombopag (EPAG), a small molecule that activates the cMPL receptor. EPAG has been found to be effective in increasing platelet levels and alleviating thrombocytopenia. We utilized computational techniques to predict and confirm the complex formed by the ligand (EPAG) and the Thrombopoietin receptor (TPO-R) cMPL, elucidating the role of RAS, JAK-2, STAT-3, and other essential elements for downstream signaling. Molecular dynamics (MD) simulations were employed to evaluate the stability of the ligand across specific proteins, showing favorable characteristics. For the first time, we examined the presence of TPO-R in human umbilical cord mesenchymal stem cells (hUCMSC) and human gingival mesenchymal stem cells (hGMSC) proliferation. Furthermore, treatment with EPAG demonstrated angiogenesis and vasculature formation of endothelial lineage derived from both MSCs. It also indicated the activation of critical factors such as RUNX-1, GFI-1b, VEGF-A, MYB, GOF-1, and FLI-1. Additional experiments confirmed that EPAG could be an ideal molecule for protecting against UVB radiation damage, as gene expression (JAK-2, ERK-2, MCL-1, NFkB, and STAT-3) and protein CD90/cMPL analysis showed TPO-R activation in both hUCMSC and hGMSC. Overall, EPAG exhibits significant potential in treating radiation damage and mitigating the side effects of radiotherapy, warranting further clinical exploration.
Cite: Subbarayan R, Srinivasan D, Sadullah Usmani S, Murugan Girija D, Ikhlas S, Srivastav N, Balakrishnan R, Shrestha R, Chauhan A. Molecular insights on Eltrombopag: potential mitogen stimulants, angiogenesis, and therapeutic radioprotectant through TPO-R activation. Platelets. 2024 Dec;35(1):2359028. PMID: 38832545.