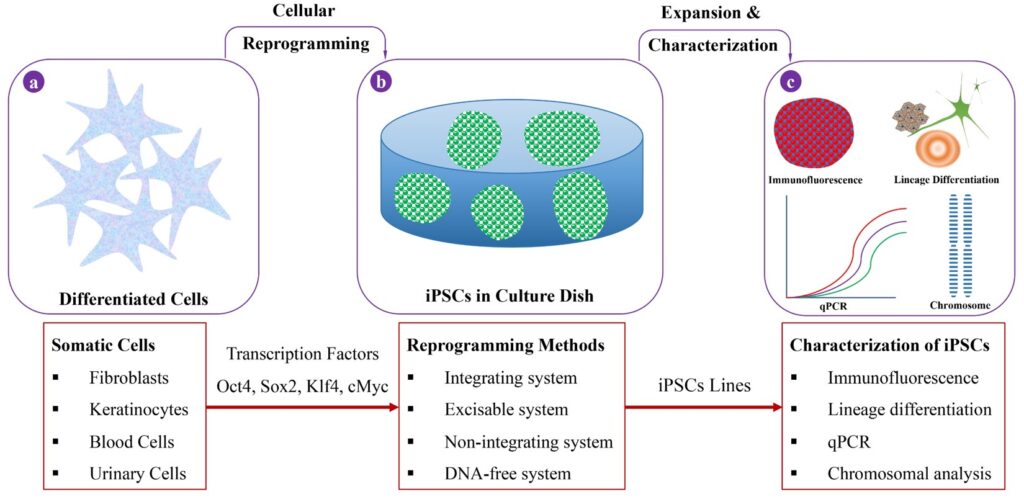
Induced pluripotent stem cells are Japanese brand sources for therapeutic cells to pretrial clinical research
Rupendra Shrestha1 2 3
- Department of Genetics, Albert Einstein College of Medicine, NY 10461, U.S
- Department of Ophthalmology and Visual Sciences, Albert Einstein College of Medicine, NY 10461, U.S
- Dr. Koirala Research Institute for Biotechnology and Biodiversity, Kathmandu, Nepal
Publication: Progress in Stem Cell. 2020 Jun; 7 (1), 296-303. doi: 10.15419/psc.v7i1.409
Abstract– iPSCs are promising and have potential benefits for medical use, understanding human organogenesis, and cell therapy for advanced diseases. iPSCs are derived pluripotent cells which can further differentiate into functional human cell-lineages, such as neuronal, epithelial cells, cardiac cell, immune cell, and blood cells, etc. Thirteen years on, the discovery of iPSC has revolutionized the field of regenerative medicine, and also the number of clinical studies using iPSC has been growing rapidly worldwide. However, Japan is leading the race of iPSC-based studies and clinical trials due to government support. The Japanese government implemented the world’s fastest approval system and set to host first pretrial clinical studies using iPSC derived therapeutic products. Also, multinational companies of Japan are investing enormously in iPSC-based research for mobilization of iPSC-derived regenerative products to the research institution globally. This review presents an overview of iPSCs, potential benefits, commercialization of iPSC, iPSC-based pretrial clinical studies, and iPSC biobanking in Japan.
Cite: Shrestha, R. Induced pluripotent stem cells are Japanese brand sources for therapeutic cells to pretrial clinical research. Progress in Stem Cell. 2020;7(1-2):296-303.