Self-formation of concentric zones of telencephalic and ocular tissues and directional retinal ganglion cell axons
- Department of Ophthalmology and Visual Sciences, Albert Einstein College of Medicine, Bronx, United States.
- Department of Genetics, Albert Einstein College of Medicine, Bronx, United States.
- The Ruth L. and David S. Gottesman Institute for Stem Cell Biology and Regenerative Medicine, Albert Einstein College of Medicine, Bronx, United States.
- Dominick P Purpura Department of Neuroscience, Albert Einstein College of Medicine, Bronx, United States.
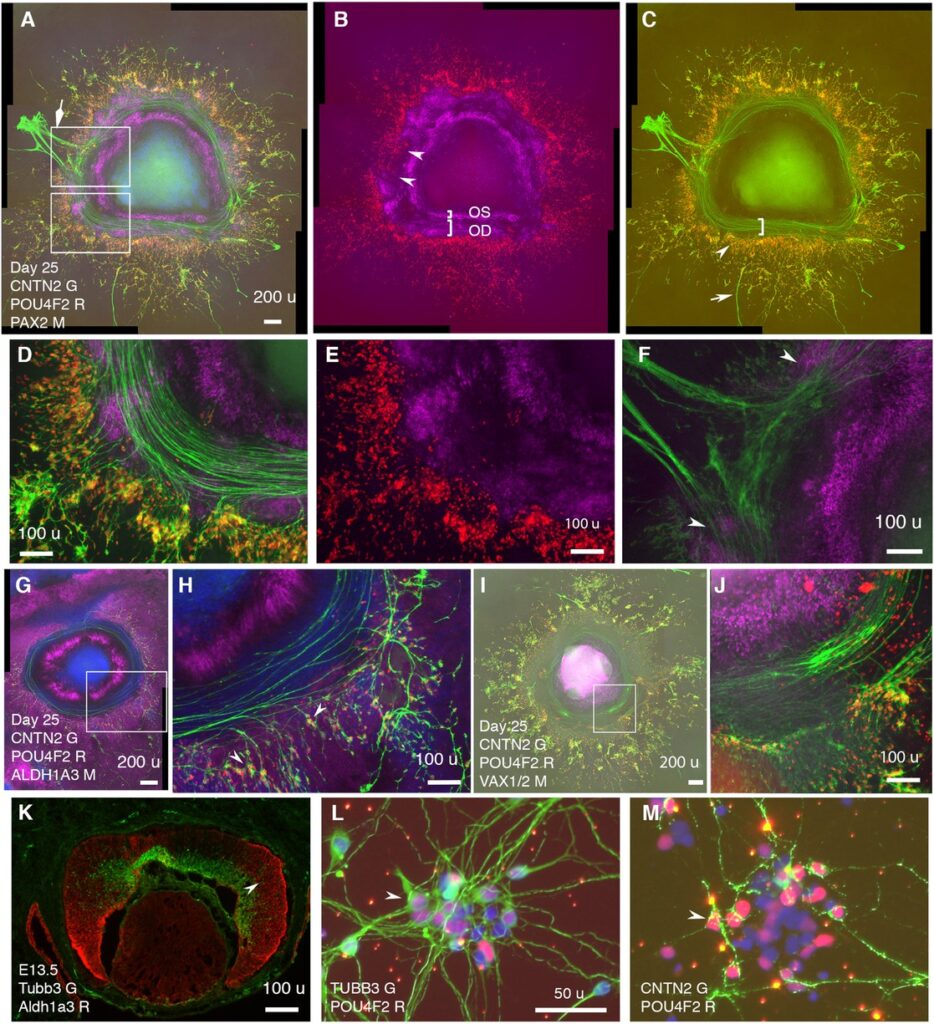
Abstract: The telencephalon and eye in mammals are originated from adjacent fields at the anterior neural plate. Morphogenesis of these fields generates telencephalon, optic-stalk, optic-disc, and neuroretina along a spatial axis. How these telencephalic and ocular tissues are specified coordinately to ensure directional retinal ganglion cell (RGC) axon growth is unclear. Here, we report self-formation of human telencephalon-eye organoids comprising concentric zones of telencephalic, optic-stalk, optic-disc, and neuroretinal tissues along the center-periphery axis. Initially-differentiated RGCs grew axons towards and then along a path defined by adjacent PAX2+ VSX2+ optic-disc cells. Single-cell RNA sequencing of these organoids not only confirmed telencephalic and ocular identities but also identified expression signatures of early optic-disc, optic-stalk, and RGCs. These signatures were similar to those in human fetal retinas. Optic-disc cells in these organoids differentially expressed FGF8 and FGF9; FGFR inhibitions drastically decreased early RGC differentiation and directional axon growth. Through the RGC-specific cell-surface marker CNTN2 identified here, electrophysiologically excitable RGCs were isolated under a native condition. Our findings provide insight into the coordinated specification of early telencephalic and ocular tissues in humans and establish resources for studying RGC-related diseases such as glaucoma.
eLife assessment: In this important study, the authors present a human telencephalon-eye organoid model that exhibits remarkable pathfinding and growth of retinal ganglion cell (RGC) axons. The identification of cell-surface markers for RGCs could have value for understanding the molecular mechanisms involved in RGC axon development and regeneration. The strength of evidence is compelling for future studies to investigate RGC neurite outgrowth and brain-eye connectivity in humans.